Harnessing life-saving chemistry with IR temperature sensors
By Joris Roels, Marketing Manager Temperature Sensors
My chemistry teacher in high school used to explain that chemical reactions can be understood as sufficiently energetic collisions between the reactants. For a reaction to happen, you need frequent collisions, and they need to have sufficient energy (activation energy). Temperature also plays a key role in chemistry, as increasing temperature essentially makes particles move faster, increasing the frequency and energy of the collisions.
Of course, every chemistry amateur knows that other factors come into play as well. Pressure, surface area (for example, a fine powder vs. a lump of material), and the concentration of the reactants will influence the frequency of collisions, while catalysts can provide alternative pathways by lowering the activation energy. However, tight temperature control is, in many cases, one of the key contributors to successful and life-saving chemistry. One such example is the well-known Polymerase Chain Reaction (PCR), which is used to amplify (duplicate) DNA. Many variants exist, but three basic steps are always present.
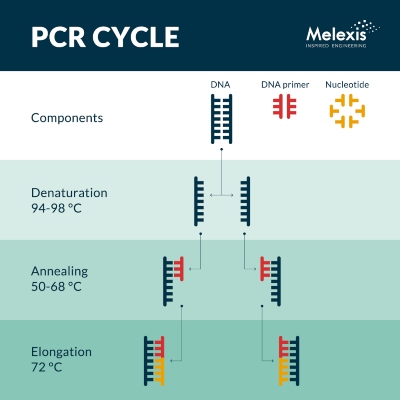 In the first high-temperature step (94-98°C) called denaturation, the DNA double helix structure is cut into two single-stranded DNA template molecules by breaking the hydrogen bonds using chemical scissors. During the next step, annealing (50-65°C), the so-called DNA primer molecules bind selectively to the two DNA templates. If the temperature is not precisely controlled, the primers may not bond in the right selective manner or at all.
In the first high-temperature step (94-98°C) called denaturation, the DNA double helix structure is cut into two single-stranded DNA template molecules by breaking the hydrogen bonds using chemical scissors. During the next step, annealing (50-65°C), the so-called DNA primer molecules bind selectively to the two DNA templates. If the temperature is not precisely controlled, the primers may not bond in the right selective manner or at all.
Finally, in elongation (typically 72°C), the nucleotides (small organic molecules that are the building blocks for DNA/RNA) in the solution react with the template and primer, resulting in double-stranded molecules. In this way, one DNA molecule is converted into two such double-stranded molecules.
The process can now be repeated, and since the material is doubled in every step, after 40 cycles, we have 2^40 = 1.099.511.627.776 molecules. Creating such a vast amount of molecules allows further detection by other means.
One of the most popular applications of PCR is detecting infection. The DNA/RNA of the pathogen, such as a virus or a bacterium, is amplified and then detected in sample from the patient. This application has boomed due to Covid-19, but the technique also allows the detection of many more disease agents.
PCR is just one example of the multiple biochemical processes that are used for medical diagnostics applications. In order to facilitate such temperature-sensitive biochemical reactions, devices called thermal cyclers are used. This apparatus contains one or multiple thermal blocks featuring holes where the tubes holding the reactants can be inserted.
The goal of the thermal cycler is to subject the tubes to a pre-determined temperature program, preferably enabling fast and accurate temperature sweeps. Some models allow a controlled temperature gradient over the thermal block, exposing different samples to different temperatures. This feature is mostly used in the research phase to optimize certain critical steps of the temperature cycle.
During testing, the specimens are replaced frequently, making it very difficult for the manufacturers to reliably measure the tubes through a direct contact method. Tight control loops rely on accurate sensor input, and that’s where infrared temperature sensors come in. They enable non-contact temperature measurement, a huge asset compared to contact thermometers. Furthermore, the risk of cross-contamination between specimens is greatly reduced by avoiding direct contact.
Factory calibrated sensors, like the MLX90614 allow easy implementation into the thermal cycler as no additional calibration step is required. A wide choice of field of view options increases the design freedom; a narrower field of view allows for a larger distance between the sensor and the sample.
Our latest innovation includes an SMD sensor (MLX90632) with a heavily reduced form factor, allowing diagnostic equipment to be smaller, lighter, and portable. Miniaturizing such sensors also facilitates integration into a sensing matrix enabling multipoint temperature control.
Medical diagnostic testing is shifting quickly from sending specimens to a specialized medical lab and waiting weeks for results towards testing at the point-of-care. Diagnostics take place virtually next to the patient, and the results are available in hours rather than days. Infrared temperature sensors play a vital but underexposed role in this unstoppable evolution. The tighter temperature control they offer allows biochemical processes to be tuned further towards faster, more accurate and more reliable diagnostics capabilities.